Molecule Madness - Activation Energy
Molecule Madness - Activation Energy
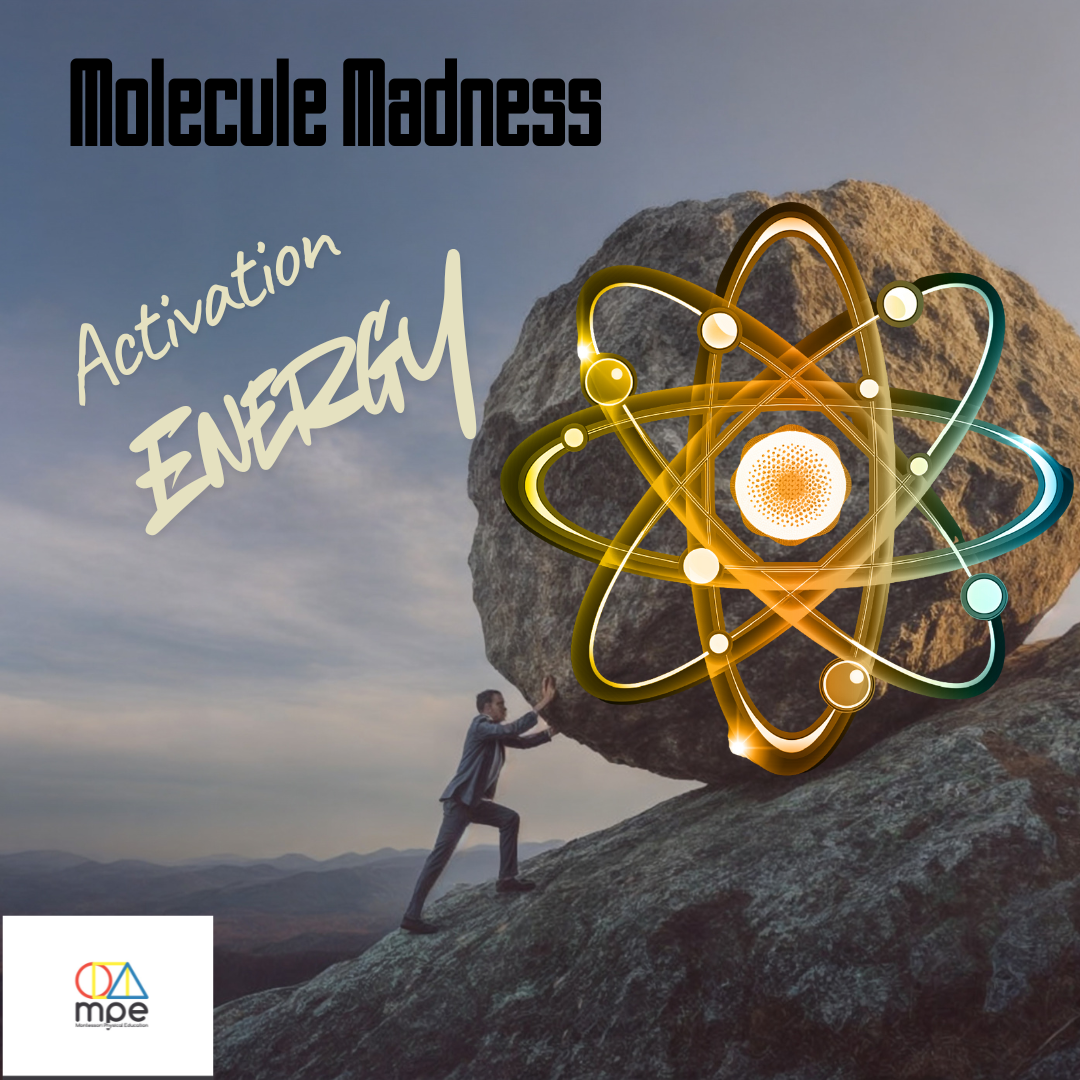
This game explores Activation Energy—the minimum energy needed for molecules to collide and react, much like the force needed to strike a match. Think of it as pushing a boulder over a hill: once pushed far enough, it rolls down, and the reaction occurs. Activation energy is the potential energy needed for molecules to reach the point of interaction, often supplied by heat through increased collisions.
In this lesson, students physically model the chemistry concept of activation energy by playing an ultimate frisbee-style game where movement, spacing, and pressure determine whether a “reaction” can occur. Players may only pivot when holding the frisbee, representing the need for molecules to orient correctly before reacting. Defensive pressure near the scoring wall represents the activation energy barrier that must be overcome for a reaction to take place.
Special Catalyst Lines on the court provide an alternative pathway. When a player catches the frisbee while standing on a Catalyst Line, they may move freely along that line, modeling how catalysts lower activation energy by offering an alternative pathway for thereaction without being consumed. A catalyst doesn’t do the work; it just lowers the activation energy required for the reaction to occur.
To better mirror real chemical reactions, every player must catch and throw the frisbee ina possession before scoring, reflecting the requirement that all reactant molecules interact before a reaction proceeds. My students suggested this rule to ensure everyone participated, and it made the game more equitable while tying it to real-life chemistry.
Materials:
• Frisbee (maybe multiple if playing in an area where one can become lost)
• Small cones or rubber disc dots
o If you are in a gym, use the court lines.
• A walled playing area (like a gym)
• Pinnies or jerseys
Minimum Number of Students Needed: This game can be played with as few as 4 players
(2V2), but ideally, you would have 12-20 students and split them evenly into 2 teams. If you
have more than that, consider halving the playing area and making two small side games.
Prior Knowledge: This lesson is intended for middle school students who are delving into
chemistry, but could be played by advanced upper elementary students.